Fang-Hui Zhao1, Jose Jeronimo2, You-Lin Qiao1, Johannes Schweizer3, Wen Chen1, Melissa Valdez2, Peter Lu3, Xun Zhang1, Le-Ni Kang1, Pooja Bansil2, Proma Paul2, Charles Mahoney3, Marthe Berard-Bergery3, Ping Bai1, Roger Peck2, Jing Li1, Feng Chen1, Mark H. Stoler4, and Philip E. Castle5
1Department of Epidemiology, Cancer Institute and Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, PR China; 2PATH, Seattle, Washington; 3Arbor Vita Corporation, Fremont, California; 4Department of Pathology, University of Virginia, Charlottesville, Virginia; and 5Global Cancer Initiative, Chester- town, Maryland
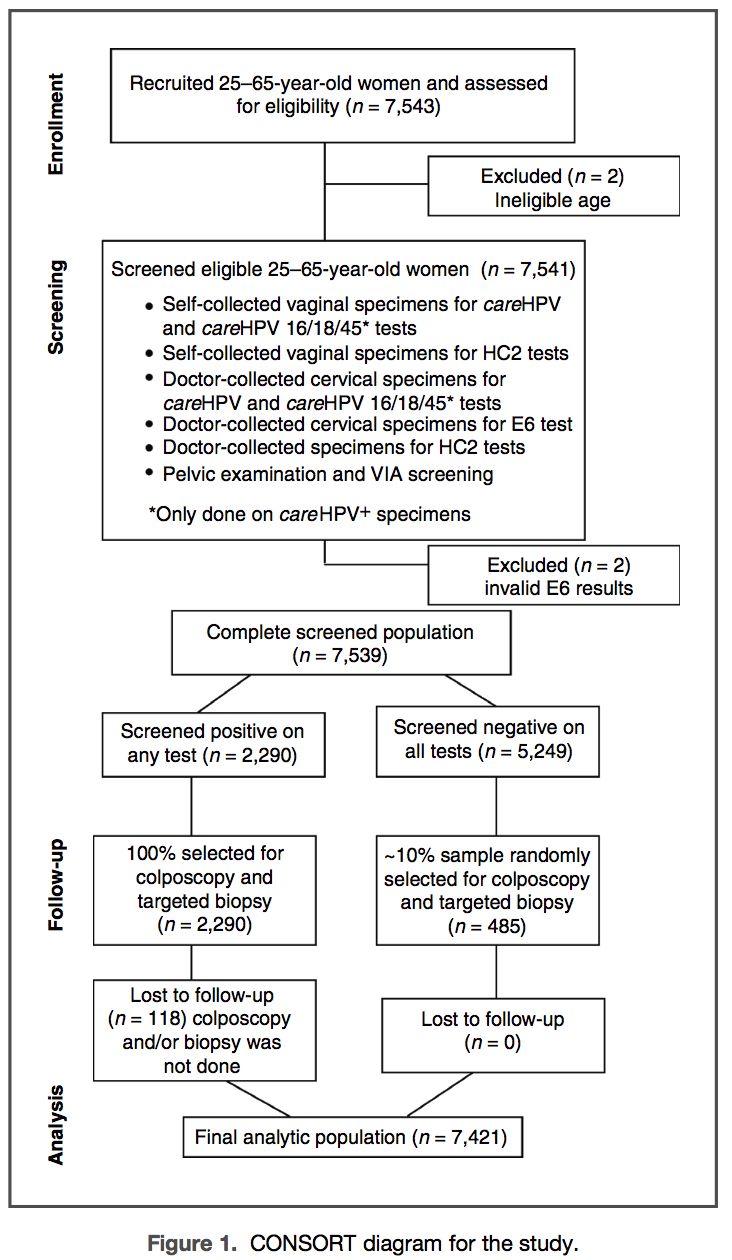
New, lower-cost tests that target high-risk human papillomavirus (HR-HPV) have been developed for cervical cancer screening in lower-resource settings but large, population-based screening studies are lacking. Women ages 25 to 65 years and living in rural China (n=7,543) self-collected a cervicovaginal specimen, had 2 cervical specimens collected by a clinician, and underwent visual inspection after acetic acid (VIA). The self- and one clinician-collected specimens underwent HR-HPV DNA testing by careHPV (QIAGEN) and Hybrid Capture 2 (HC2; QIAGEN) and the other clinician-collected specimen was tested for HPV16, 18, and 45 E6 using OncoE6 (Arbor Vita Corporation). Women who screened positive for any test and a random sample of those negative on all tests underwent colposcopic evaluation. The percent test positive was 1.8% for HPV E6 oncoprotein, between 14% and 18% for HR-HPV DNA testing, and 7.3% for VIA. The sensitivity for cervical intraepithelial neoplasia grade 3 or more severe (CIN3+; n =99) was 53.5% for OncoE6, 97.0% for both careHPV and HC2 testing of the clinician-collected specimen, 83.8% for careHPV testing and 90.9% for HC2 testing of the self-collected specimen, and 50.5% for VIA. OncoE6 had the greatest positive predictive value (PPV), at 40.8% for CIN3+, compared with the other tests, which had a PPV of less than 10%. OncoE6 tested 70.3% positive for HPV16, 18, or 45-positive CIN3+ and tested negative for all HPV16-, 18-, or 45-negative CIN3+(P < 0.0001). HPV E6 oncoprotein detection is useful for identifying women who have cervical precancer and cancer. Cancer Prev Res; 6(9); 938–48. ©2013 AACR.

